In pharmaceuticals research, production and marketing there may be errors from the human side or due to machine error. Despite the tedious approach to omit these errors, they are likely to happen due to some or the other reasons. Hence, we need a check and improve plan to minimize the negative effects of these errors. So, for this purpose there comes CAPA in pharmaceuticals.
What Are Corrective and Preventive Actions (CAPA)?
Corrective and preventive actions (CAPA) in pharmaceuticals is a quality approach that deals with the identification, collection, analyzation and execution of preventive plan to minimize the expected side effects due to errors that took place during the pharmaceutical process. CAPA aids to eradicate nonconformities within the organization.
Difference Between Corrective And Preventive Actions:
The difference between the corrective and preventive action is mainly upon their intention to solve the non-conformities which is explained below:
Importance Of CAPA In Regulatory Industries:
CAPA plays a vital role in the pharmaceutical industry as it prevents errors and safeguards the safety and quality issues and makes sure that the industry works under regulatory compliance. Some of these key features are explained below:
Quality Of Product:
CAPA helps in achieving quality in product by implementing corrective measures and by rectifying the root cause of problem that is causing quality issues.
Determination Of Root Cause:
With the help of CAPA one can identify the root causes that are causing errors in the pharmaceutical company and hence by rectifying it an errorless working can take place in pharmaceutical company.
Analysis Of Root Cause:
By analyzing the root cause CAPA helps one to understand the reason for the problem and what measures can be taken to improve and avoid the problem.
Check On Good Manufacturing Practices:
A certain set of practices that are covered under good manufacturing practices to ultimately result in a good pharmaceutical product needs to be kept monitoring which is facilitated by CAPA.
Audit Of Manufacturing Process:
Frequent auditing helps the pharmaceutical company to achieve a successful product, thus CAPA helps in this auditing by deeply analyzing each step of the pharmaceutical process.
CAPA Implementation Strategy:
After determining the actual corrective actions that must be taken, it gets easy to understand and make a strategy for implementation.
Regulatory Compliance:
CAPA helps in complying with the regulatory requirements by identifying the issues in meeting the compliance and providing strategies to meet them.
Achieving Consumer Trust:
After a successful pharmaceutical product which is achieved due to CAPA, customer trust is easily built up which eventually increases the market value of the product.
How To Perform CAPA?
CAPA Regulatory Requirements:
CAPA should follow regulatory standards such as ISO 9001, ICH Q10, FDA 21 CFR PART 820. Some of their key requirements are mentioned below:
FDA 21 CFR Requirements:
- Analyze data from the complaints, audits, non-conformities, etc.
- Investigate the root cause of the complaint, non-conformities, etc. And document it.
- Plan corrective action according to the data and set strategies to implement them.
- Validate the corrective and preventive actions.
- Document the whole CAPA process and keep monitoring it.
ISO 9001:2005 And ISO 13485 Requirements:
- Identify the non-conformities.
- Address them by corrective actions.
- Audit and monitor the CAPA.
- Implement strategies for reoccurrence of root causes.
ICH Q10 Requirements:
- Data should be collected from various sources.
- Determine the root cause.
- Focus on risk-based approach.
- Enforce and overview corrective plans
- Enforce and overview preventive plans.
- Take measures for continuous improvement.
- Document the whole process of CAPA.
CAPA Steps For An Effective Process:
There are a certain set of steps of CAPA which are mentioned below in their sequence.
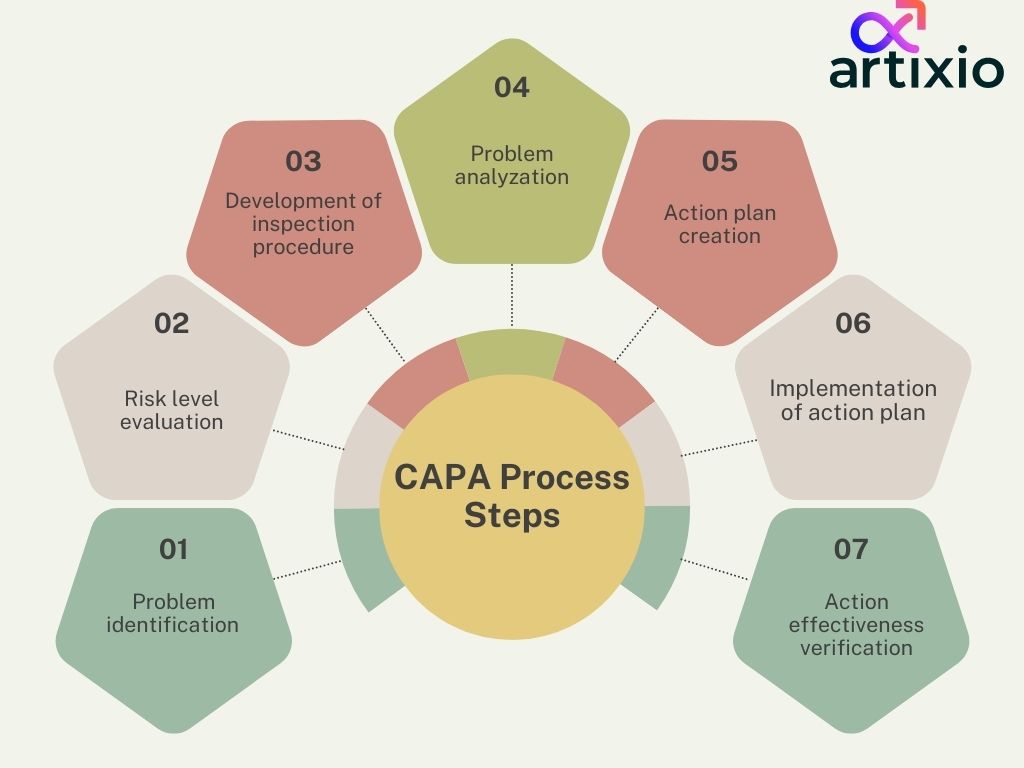
- Problem Identification: This is the first step of CAPA process which involves the actual problem identification.
- Risk level Evaluation: After the identification of problems evaluate the risk level of the problem.
- Development Of Inspection Procedure: Then develop a plan for inspection procedure.
- Problem analyzation: Perform detailed analyzations of problems.
- Action Plan Creation: Create an action plan to eradicate the problem and its root cause.
- Implementation Of Action Plan: Implement the action plan.
- Action Effectiveness Verification: Conduct verification after implementation to confirm the effectiveness of action plan.
FDA Expectation From Company’s CAPA Process:
The company preparing a CAPA document should meet the following requirements of the FDA:
- The company should verify the CAPA procedure in terms of quality management.
- The sources of the non-conformities should be accurate, and the non-conformity should be true.
- The company should identify potential products and actual problems that need to be addressed by a corrective action plan.
- They should verify the data of complaints and non-conformities.
- There should be verification of the data with another set of similar data.
- The company should follow failure investigation procedure.
- Determination of appropriate corrective and preventive actions should be made.
- The effectiveness of the CAPA should be checked.
- Validation of CAPA should be done.
- Systematic documentation should be done.
- The CAPA plan should be systematically implemented.
FAQs About CAPA
Q1: Explain the difference between corrective and preventive actions?
Corrective actions deal with problems that have already occurred, whereas preventive actions deal with identifying potential future issues.
Q2: What are key elements of CAPA process?
The key elements of CAPA include; issue identification, root cause analysis, corrective/preventive action planning, implementation, and effectiveness verification.
Q3: Why do regulatory authorities focus on compliance of CAPA?
Regulatory authorities focus on CAPA compliance as manufacturers address quality issues promptly and proactively, reducing risks to public health and improving product safety.
Q4: What are common mistakes in the CAPA process?
Root cause analysis, failure to documentation and lack of follow-up on effectiveness monitoring are some of the common CAPA process mistakes.





